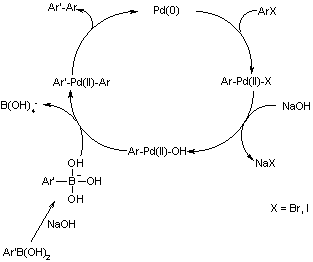
Ligand-Free Palladium Catalysis of the Suzuki Reaction in Water Using Microwave Heating | Organic Letters

Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Esters | Journal of the American Chemical Society
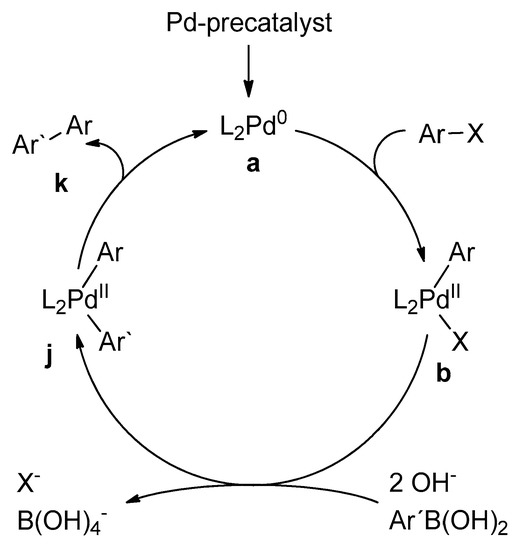
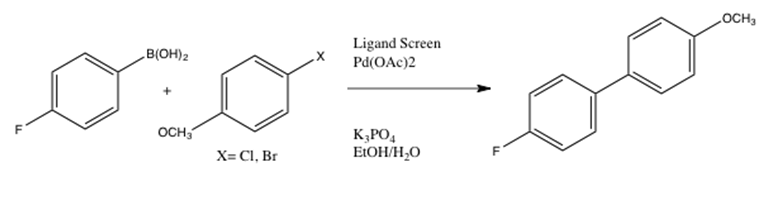
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross- Coupling Reactions: Generation of Carbon–Carbon Bond

Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Esters | Journal of the American Chemical Society

Palladium-catalyzed, ligand-free Suzuki reaction in water using aryl fluorosulfates. | Semantic Scholar

Base-Free Suzuki–Miyaura Coupling Reaction Using Palladium(II) Supported Catalyst in Water | Catalysis Letters
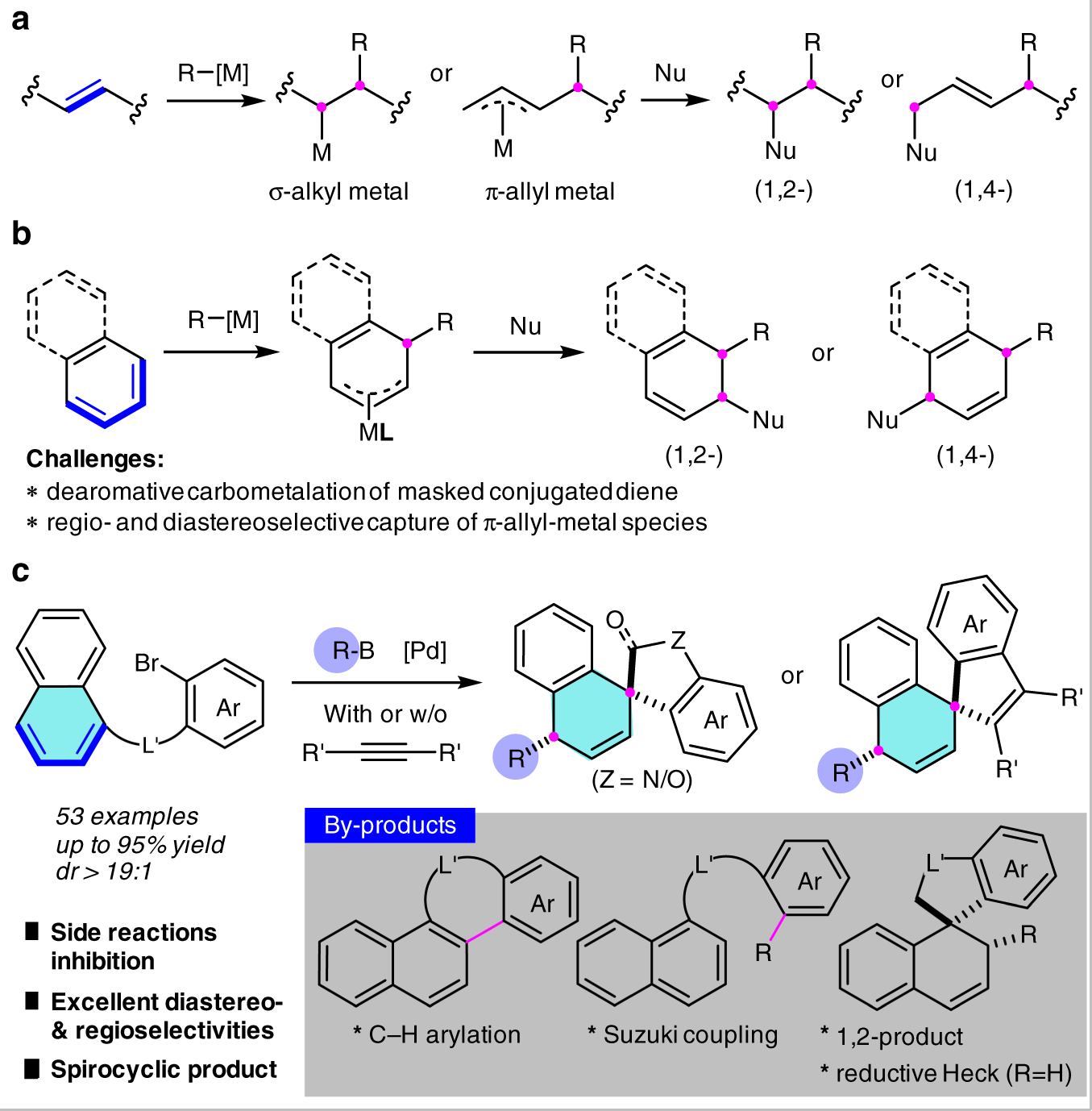
Dearomative 1,4-difunctionalization of naphthalenes via palladium-catalyzed tandem Heck/Suzuki coupling reaction | Nature Communications

Palladium nanoparticles catalyzed Suzuki cross-coupling reactions in ambient conditions - ScienceDirect

Suzuki–Miyaura cross-couplings for alkyl boron reagent: recent developments—a review | Future Journal of Pharmaceutical Sciences | Full Text
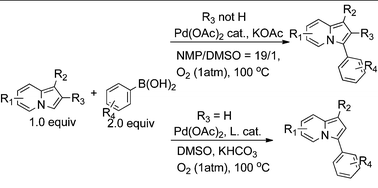
Palladium catalyzed oxidative Suzuki coupling reaction of indolizine at the 3-position using oxygen gas as the only oxidant - RSC Advances (RSC Publishing)

Homogeneous and Recyclable Palladium Catalysts: Application in Suzuki–Miyaura Cross-Coupling Reactions | Organometallics

Base-Free Suzuki–Miyaura Coupling Reaction Using Palladium(II) Supported Catalyst in Water | Catalysis Letters

Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Esters | Journal of the American Chemical Society